Integrated Care Stories
Ensuring vaccine safety
Kaiser Permanente achieves better outcomes through a combination of care coordination, comprehensive data collection and use, and aligned incentives that all promote affordable, high-quality care. This case study, part of our Integrated Care Stories series, highlights the benefits of Kaiser Permanente’s approach.
The challenge
Vaccines protect people from harmful diseases such as measles, mumps, rubella, diphtheria, and tetanus. They reduce the severity of disease and decrease hospitalizations, deaths, and other negative outcomes. Large numbers of people getting vaccinated also reduces disease transmission rates, which improves population health. Vaccines for many diseases have been available for decades, but some, such as the vaccines for the HPV and COVID-19, were developed more recently.
Before they’re approved for broad use, vaccines go through a rigorous review process, including extensive testing among large numbers of people. Even after approval, the Centers for Disease Control and Prevention and other agencies closely track outcomes. Careful monitoring and open communication about any potential side effects ensures vaccine safety and increases people’s confidence in vaccines. The CDC monitors vaccine safety in several ways,1 and one of these approaches requires partnership with integrated health care organizations.
The integrated care and coverage solution
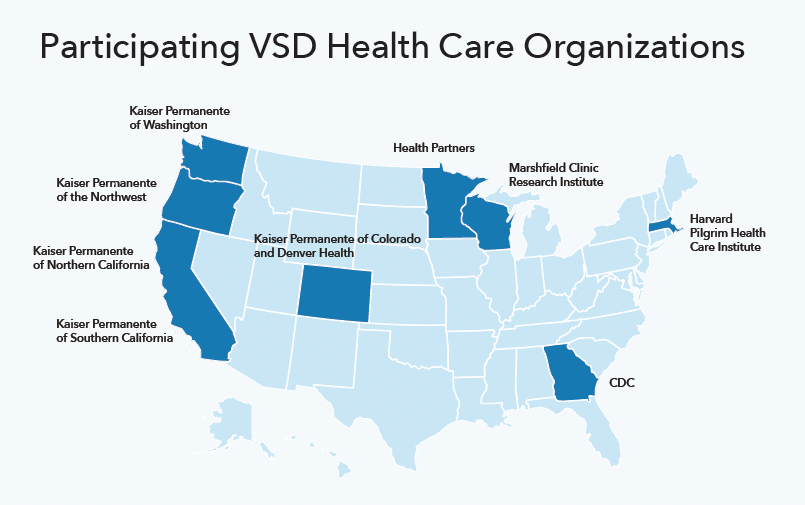
Since 1990, the CDC has partnered with several integrated health care organizations, including Kaiser Permanente, to track outcomes among large numbers of patients who receive a vaccine after approval. Tracking outcomes helps the CDC identify any rare side effects not discovered during extensive preapproval testing. The collaborative, called the Vaccine Safety Datalink project (VSD), uses secure, privacy-protected data, with Kaiser Permanente representing most of the data.
The VSD uses electronic health records instead of self- or physician-reported data to track vaccine safety after approval. This makes VSD studies faster and more complete than many of the other post-approval approaches for monitoring vaccine safety. Each participating VSD organization keeps its own secure data and maintains that data in a consistent way. This allows analyses to be aligned across sites while maintaining patient data privacy and security.
In one current VSD study, researchers at Kaiser Permanente have been partnering with the CDC to monitor the safety of COVID-19 vaccines. Based on an analysis of the health data of 6.2 million vaccinated people from VSD organizations, the research team confirmed that no serious health problems resulted from mRNA COVID-19 vaccines.2
How Kaiser Permanente’s integrated care and coverage model supported this innovation
Extensive population-level data
With access to data across large numbers of patients over long periods of time, we track population health trends and identify and pursue opportunities to improve and eliminate disparities in care. Our extensive database and our research capacities allow us to serve as a helpful clinical and health services research partner to national agencies and academic institutions.
As an integrated health care organization, we can scan our vast, secure health record databases to ensure vaccine safety. We gather information about the health of large numbers of people who received a particular vaccine. We then use statistical techniques to analyze anonymized and aggregate data, looking for patterns over time and comparing health trends among members.
This population health approach allows VSD researchers to identify potential serious health problems, even if extremely rare, that could be associated with a vaccine. Our population-level data allows us to provide national agencies like the CDC with uniquely valuable information as they carry out their role of ensuring public safety and promoting public health.
Research and quality improvement
We analyze care management processes and outcomes and use the results to continuously improve. We then share findings from this practice-based clinical research to benefit others beyond Kaiser Permanente.
The VSD enables population-based vaccine safety evaluations and also has been used to understand the demographic characteristics of people receiving various vaccines, disease incidence, and cost-effectiveness.3 VSD findings are shared in presentations across the country and published in a wide range of medical journals.4
We also participate in CISA, the CDC’s Clinical Immunization Safety Assessment project, which coordinates a national network of vaccine safety experts. CISA conducts clinical research, provides consultations to U.S. clinicians and public health partners, and develops evidence-based guidelines for clinicians.5
Focus on individual and population health
Our mission, and our long-term perspective — free from investors’ focus on short-term returns — motivates us to address individual and community drivers of health. We analyze member health trends to identify opportunities for improvement and support quality improvement and prevention. Our investments in individual and population health help us retain our members, and we benefit when those long-tenured members and the broader communities we serve realize positive health outcomes.
The VSD project allows researchers to help ensure vaccine safety by conducting post-approval, population-based reviews of vaccine outcomes. Only a few programs in the world conduct these kinds of analyses,6 so VSD studies provide important information about vaccine safety that might not otherwise be available. This information benefits our members, the communities we serve, and the broader public.
Kaiser Permanente’s integrated care and coverage model differs from much of the United States health care system, which relies on disconnected clinical practices paid through fee-for-service. These disconnected approaches typically result in highly fragmented care, a focus on quantity of services rather than high-quality care, and an emphasis on acute care rather than long-term investments in population and community health.
Kaiser Permanente achieves better outcomes through a combination of care coordination, comprehensive data collection and use, and aligned incentives that all promote affordable, high-quality care. This case study, part of our Integrated Care Stories series, highlights the benefits of Kaiser Permanente’s approach.
Closing the vaccination gap
A panel of business leaders discuss how the private sector can work together to close the vaccination gap and end the COVID-19 pandemic.
The Vaccine Safety Datalink (VSD)
The Vaccine Safety Datalink (VSD) is a collaborative project between CDC’s Immunization Safety Office, integrated health care organizations, and networks across the U.S.
Kaiser Permanente’s vaccine study center
We help protect public health with the following programs.
- Ensuring Vaccine Safety U.S. Centers for Disease Control and Prevention. Vaccine Safety Monitoring. Accessed October 27. 2021 from https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/index.html. U.S. Department of Health and Human Services. Vaccine Safety. Accessed October 27. 2021 from https://www.hhs.gov/immunization/basics/safety/index.html.
- Klein, Nicola P, Lewis, Ned, Goddard, Kristin, et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA. 2021;326(14):1390-1399. doi:10.1001/jama.2021.15072.
- James Baggs, et al., “The Vaccine Safety Datalink: A Model for Monitoring Immunization Safety,” Pediatrics, May 1, 2011, https://publications.aap.org/pediatrics/article/127/Supplement_1/S45/30123/The-Vaccine-Safety-Datalink-A-Model-for-Monitoring?autologincheck=redirected.
- Centers for Disease Control and Prevention. Vaccine Safety Datalink Publications. Accessed October 29, 2021 from https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/publications.html.
- Kaiser Permanente Division of Research, “Vaccine Study Center,” November 24, 2021, https://divisionofresearch.kaiserpermanente.org/vaccineresearch/vaccinestudycenter/whatwedo; and Centers for Disease Control and Prevention, “Clinical Immunication Safety Assessment (CISA) Project, November 24, 2021, https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
- James Baggs, et al., “The Vaccine Safety Datalink: A Model for Monitoring Immunization Safety,” Pediatrics, May 1, 2011, https://publications.aap.org/pediatrics/article/127/Supplement_1/S45/30123/The-Vaccine-Safety-Datalink-A-Model-for-Monitoring?autologincheck=redirected.