Integrated Care Stories
Improving patient outcomes and promoting medical device safety
Kaiser Permanente achieves better outcomes through a combination of care coordination, comprehensive data collection and use, and aligned incentives that all promote affordable, high-quality care. This case study, part of our Integrated Care Stories series, highlights the benefits of Kaiser Permanente’s approach.
The challenge
Millions of Americans rely on medical devices to provide life-sustaining care and enhance their quality of life. Medical devices include products that replace joints, deliver medications, and monitor or support critical bodily functions like hearing and cardiovascular activity. Common medical devices include nebulizers that turn liquid medications into an inhalable mist for conditions like asthma, and surgical implants like cardiac pacemakers and prosthetic joints.
Medical devices improve patient health, but they also come with risks. Over the last decade, more than 1.7 million injuries and 83,000 deaths in the U.S. have been linked to medical devices.1
The United States Food and Drug Administration requires premarket testing and approval for most surgical implants, a specific type of medical device, but this has inherent challenges due to the limited duration of premarket testing and the types of clinical outcomes that can be measured during that time.2;3
After approval, safety concerns may emerge as the devices are used with larger numbers of people and over longer time periods. Most post-market monitoring, however, is voluntary. The FDA believes there is substantial under reporting of device-related problems, making it challenging to evaluate device safety and performance across more than 6,500 medical device companies.4;5 When devices are recalled, manufacturers are not required to notify patients directly but instead typically mail limited information about the recall to health care providers.6;7 Providers must evaluate the implications for their patients and may not have sufficient information to identify and contact all affected individuals.8;9

The integrated care and coverage solution
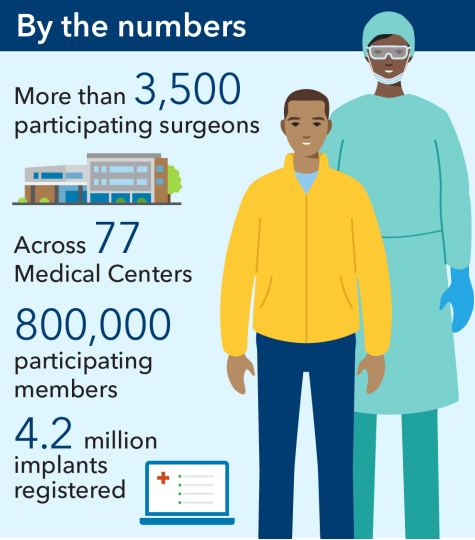
In 2001, a team at Kaiser Permanente launched a registry to monitor and evaluate prosthetic joints and surgical techniques used in knee and hip replacements. Kaiser Permanente surgeons and registry team members designed standardized documentation forms to collect data on surgical techniques, patient and prosthesis characteristics, and clinical outcomes. They then used these data to identify and implement best practices for hip and knee procedures.10 As a result, patients reported less pain after surgery and were less likely to need reoperation, saving them unnecessary discomfort and expense and reducing costs for Kaiser Permanente by more than half a million dollars in just the first 6 years.11
Kaiser Permanente’s Medical Device Surveillance and Assessment program has since expanded to include registries for anterior cruciate ligament knee (ACL) reconstruction, and shoulder, hip fracture, spine, cardiac, and vascular devices. Our registry teams encourage clinicians to both enroll their patients in our registries and actively follow up with them over time, resulting in high participation rates – above 95% for many of our registries.12 We are currently monitoring over 4.2 million medical devices.13 Our registries influence clinical practices and device selection across our medical centers. They inform innovative programs like our National Total Joint Replacement Initiative, which has shortened hospital length of stay by 80% and reduced costs by approximately $214 million.14;15
Thanks to our registries, we have improved care for our members and patients across the country. For example, our registries team identified 3 underperforming stents used in endovascular aortic repair, or EVAR, that were associated with higher risk of life-threatening leaks and ruptures.16 We discontinued use of these stents within Kaiser Permanente, developed device-specific processes for monitoring patients after EVAR, and provided evidence that informed the FDA’s decision to recall an EVAR stent device.
The FDA later collaborated with Kaiser Permanente to develop a national long-term monitoring and assessment network for EVAR devices, which will promote better outcomes for all patients.17
How Kaiser Permanente’s integrated care and coverage model supported this innovation
Extensive population-level data:
With access to data across large numbers of patients over long periods of time, Kaiser Permanente tracks population health trends, and identifies and pursues opportunities to improve and to eliminate disparities in care. Our extensive database and our research capacities allow us to serve as a helpful clinical and health services research partner to national agencies and academic institutions.
Care teams use evidence from our registries to improve surgical techniques and post-operative care. For example, uncemented fixation has traditionally been the preferred approach to partial hip replacement in the U.S. However, Kaiser Permanente registry findings highlighted the benefits of cemented fixation, with half the risk of revision compared to uncemented fixation.18 This has already begun to influence clinical practice across Kaiser Permanente.
Our registries help us identify and address disparate outcomes among our patients, improving patient health and experience. For example, we have identified patients and implants at higher risk for revision surgery after ACL reconstruction and worked with our surgical teams to improve the selection of grafts used in ACL reconstruction.19 This led to substantially lower rates of revision surgery.20 Our registries also support better health for patients outside of our clinics, as we alert the FDA about devices that are underperforming and could pose risks. Our National Implant Registries team also partnered with Cornell University to develop FDA US-based performance standards for total joint device evaluations.21
High-quality care with clinical decisions made by doctors and patients:
Physicians work with their patients to develop personalized care plans, making decisions about medical care together. To inform those decisions, doctors use the best available evidence and create clinical practice guidelines. They champion quality improvement, ensuring that our care teams practice medicine based on the best, most up-to-date information, incorporating innovative approaches.
Our patients and physicians work together to make informed, personalized decisions about medical devices and surgical techniques, determining which options are best for each patient’s needs based on robust evidence from our registries and research.
Our support continues long after patients begin using medical devices. The FDA can recall medical devices when evidence suggests they could cause serious health problems, but it is not always easy for patients to learn whether their device has been recalled. Using our registries, Kaiser Permanente clinicians can quickly identify affected patients and partner with them to develop timely solutions based on each patient’s needs and preferences and the most up-to-date data on alternatives. In 2022, more than 15,000 Kaiser Permanente patients were notified about a recall, supported with enhanced monitoring, and advised on evidence-based next steps to keep them healthy and safe.22
Research and quality improvement:
We analyze care management processes and outcomes and use the results to continuously improve. We then share findings from this practice-based clinical research to benefit others beyond Kaiser Permanente. In addition, our care teams can access a constantly updated clinical library, helping us stay abreast of additional new evidence-based approaches.
At Kaiser Permanente, we use evidence from our registries to establish best practices and equip all our care teams with the data they need to improve quality. We produce benchmarking reports that support individual medical centers and surgeons to provide high-quality care and collaborate on quality improvement. These reports also enable us to identify and address regional variations in practice.23 Our clinicians and analysts work together to answer the most pressing questions about medical devices, surgical techniques, and care quality on behalf of patients everywhere.
Our registry teams have produced over 250 research publications and 460 conference presentations on clinical best practices, cost effectiveness, and patient safety.24
We have collaborated with health systems and registry teams in other countries as they aim to develop similarly innovative approaches to monitoring and researching medical devices and surgical techniques.
Kaiser Permanente’s registry team has been awarded the Orthopaedic Research and Educational Foundation Clinical Research Award for Outstanding Orthopaedic Research, the Eisenberg Award for Quality and Patient Safety from the Joint Commission and National Quality Forum, and multiple American Academy of Orthopaedic Surgeons Awards of Excellence.25
Flexibility within a budget:
The Kaiser Foundation Health Plan collects member premiums in advance and then pays delivery system partners for members’ care throughout the year. This creates the flexibility within and across the health plan, the hospitals, and the medical groups to deploy those resources to best meet our members’ needs and encourages wise use of resources. Instead of relying on revenues generated when patients are sick, we are incentivized to invest in longer-term infrastructure and population health investments that improve long-term outcomes, and to provide timely, effective, and efficient care and service that allow us to retain and expand our membership.
Integrating medical device registries and research into our health system has led to substantially better patient outcomes as well as cost savings. For example, using evidence from our total joint replacement registry and feedback from patients, our physician-driven National Total Joint Replacement Initiative identified best practices for surgical and anesthesia techniques, patient education, and discharge planning.26 Using this information, they developed a home recovery program that kept joint replacement patients safe and enabled them to return home the same day of their surgery.27
This initiative increased patient satisfaction and reduced hospital readmission rates, saving an estimated $72 million in its first 3 years, and also reduced hospital length of stay by 80%.28;29 Since then, our teams have used evidence from our registries to expand the total joint home recovery program to more patients, resulting in a 75% increase in same-day discharge and estimated cost savings of more than $214 million.30;31;32
Kaiser Permanente’s integrated care and coverage model differs from much of the United States health care system, which relies on disconnected clinical practices paid through fee-for-service. These disconnected approaches typically result in highly fragmented care, a focus on quantity of services rather than high-quality care, and an emphasis on acute care rather than long-term investments in population and community health.
Kaiser Permanente achieves better outcomes through a combination of care coordination, comprehensive data collection and use, and aligned incentives that all promote affordable, high-quality care. This case study, part of our Integrated Care Stories series, highlights the benefits of Kaiser Permanente’s approach.
For an example of how our registries help our members live healthy, fulfilling lives, watch Paul Canter tell his story.
- “Medical Devices Harm Patients Worldwide As Governments Fail On Safety,” International Consortium of Investigative Journalists, https://www.icij.org/investigations/implant-files/medical-devices-harm-patients-worldwide-as-governments-fail-on-safety/, accessed January 12, 2024.
- “Premarket Approval (PMA) Review Process,” United States Food and Drug Administration, https://www.fda.gov/medical-devices/premarket-approval-pma/pma-review-process, accessed January 12, 2024
- Jonathan J. Darrow, SJD, LLM, JD, MBA et al., “FDA Regulation and Approval of Medical Devices: 1976-2020,” JAMA, August 3, 2021, p. 420.
- “Medical Device Industry Facts,” Advanced Medical Technology Association, https://www.advamed.org/medical-device-industry-facts/#:~:text=Medical%20technology%20innovators%20are%20committed,create%20medical%20miracles%20every%20day., accessed January 12, 2024.
- “Medical Device Reporting (MDR): How to Report Medical Device Problems,” United States Food and Drug Administration, https://www.fda.gov/medical-devices/medical-device-safety/medical-device-reporting-mdr-how-report-medical-device-problems, accessed January 12, 2024.
- “Recalls, Corrections and Removals (Devices),” United States Food and Drug Administration, https://www.fda.gov/medical-devices/postmarket-requirements-devices/recalls-corrections-and-removals-devices, September 29, 2020.
- “Medical Device Recalls: Patient-Focused Communications,” United States Food and Drug Administration, https://www.fda.gov/media/152470/download, October 6 2021.
- “Initiation of Voluntary Recalls Under 21 CFR Part 7, Subpart C” United States Food and Drug Administration, https://www.fda.gov/media/123664/download, March 2022.
- Zipp, Ricky, “Anatomy of a Medical Device Recall,” MedTech Dive, https://www.medtechdive.com/news/medical-device-recall-process-fda-philips-medtronic/608205/, October 18, 2021.
- Elizabeth W Paxton, PhD, MA, et al., “The Kaiser Permanente implant registries: effect on patient safety, quality improvement, cost effectiveness, and research opportunities,” Permanente Journal, Spring 2012, p. 3
- Elizabeth W Paxton, PhD, MA, et al., “The Kaiser Permanente National Total Joint Replacement Registry,” Permanente Journal, Summer 2008, p. 12.
- Elizabeth W Paxton, PhD, MA, et al., “The Kaiser Permanente National Total Joint Replacement Registry,” Permanente Journal, Summer 2008, p. 12.
- 2022 Annual Report, Kaiser Permanente Medical Device Surveillance and Assessment, 2023.
- Nithin C. Reddy, MD, et al., “Frequency and Timing of Complications and Catastrophic Events After Same-Day Discharge Compared With Inpatient Total Hip Arthroplasty,” Journal of Arthroplasty, July 2021.
- 2022 Annual Report, Kaiser Permanente Medical Device Surveillance and Assessment, 2023.
- Heather A. Prentice, PhD, MPH, et al., “Risk for surgical interventions following endovascular aneurysm repair with Endologix AFX or AFX2 Endovascular AAA Systems compared with other devices,” Journal of Vascular Surgery, August 2023, p. 333.
- “Medical Device Surveillance and Assessment Newsletter,” Kaiser Permanente, November 2023.
- Kanu Okike, MD, MPH, et al., “Association Between Uncemented vs Cemented Hemiarthroplasty and Revision Surgery Among Patients With Hip Fracture,” JAMA, March 17, 2023, p. 1077.
- Gregory B. Maletis, MD, et al., “Optimizing anterior cruciate ligament reconstruction: Individualizing the decision-making process using data from the Kaiser Permanente ACLR Registry: 2018 OREF award paper,” Journal of Orthopaedic Research, March 10, 2021, p. 29.
- Gregory B. Maletis, MD, et al., “An Interrupted Time Series Analysis Measuring the Impact of Research and Education on Clinical Practice: Decreasing Allograft Use in Young Patients Using a Registry to Track Outcomes,” Journal of Bone and Joint Surgery, April 19, 2023, p. 614.
- 2020 Annual Report, Kaiser Permanente Medical Device Surveillance and Assessment, 2021.
- 2022 Annual Report, Kaiser Permanente Medical Device Surveillance and Assessment, 2023.
- Elizabeth W Paxton, PhD, MA, et al., “The Kaiser Permanente implant registries: effect on patient safety, quality improvement, cost effectiveness, and research opportunities,” Permanente Journal, Spring 2012, p. 36.
- 2022 Annual Report, Kaiser Permanente Medical Device Surveillance and Assessment, 2023.
- 2021 Annual Report, Kaiser Permanente Medical Device Surveillance and Assessment, 2022.
- Janet Byron, “Permanente physicians lead charge to reduce overnight stays after joint replacement,” The Permanente Federation, January 20, 2021, https://permanente.org/permanente-physicians-lead-charge-to-reduce-overnight-stays-after-joint-replacement/.
- Kate E. Koplan, MD, MPH, et al., “Same-Day Joint Replacement Care: Achieving the Quadruple Aim,” NEJM Catalyst, January 20, 2021.
- Nithin C. Reddy, MD, et al., “Frequency and Timing of Complications and Catastrophic Events After Same-Day Discharge Compared With Inpatient Total Hip Arthroplasty,” Journal of Arthroplasty, July 2021.
- Margaret C. Wang, PhD, MPH, et al., “Factors Influencing Patient Satisfaction With Care and Surgical Outcomes for Total Hip and Knee Replacement,” Permanente Journal, November 3, 2021.
- Michael Hachadorian, MD, et al., “Association between same-day discharge shoulder arthroplasty and risk of adverse events in patients with American Society of Anesthesiologists classification ≥3: a cohort study,” Journal of Shoulder and Elbow Surgery, November 2023, p. e556.
- Nithin C. Reddy, MD, et al., “Association Between Same-Day Discharge Total Joint Arthroplasty and Risk of 90-Day Adverse Events in Patients with ASA Classification of ≥3,” Journal of Bone and Joint Surgery (American Volume), November 3, 2021, p. 2032.
- 2022 Annual Report, Kaiser Permanente Medical Device Surveillance and Assessment, 2023.